Focal Segmental Glomerulosclerosis (FSGS) is a serious and rare disease that attacks the kidney’s filtering units, the glomeruli.
DMX-200 is a chemokine receptor (CCR2) blocker and is administered to patients taking an angiotensin II type I (AT1) receptor blocker (ARB), which the standard of care treatment for kidney disease. DMX-200 has granted patents in various territories until 2032, with patent applications in that may extend this to 2042 if granted.
Successful Phase 2 Study
In 2020, DMX-200 demonstrated clear benefit to patients with FSGS in its first Phase 2a study in patients specifically with FSGS, following several successful studies in patients with a range of Chronic Kidney Diseases. All trial endpoints were achieved, and DMX-200 was determined to be safe and tolerable.
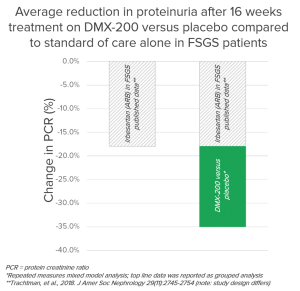
The common clinical marker for progression of kidney disease is an increase in protein in the urine (known as proteinuria), and a significant reduction in proteinuria demonstrates the progression of kidney failure as been slowed.
In the study:
- 86% of patients demonstrated reduced proteinuria on DMX-200 versus placebo
- 29% of patients demonstrated >40% reduction in proteinuria
Noting that this result was in addition to the background therapy of an ARB.