The purpose of the ACTION3 study is to determine whether the investigational medication DMX-200 is effective and safe in treating a rare kidney condition called Focal Segmental Glomerulosclerosis (FSGS). Dimerix is currently enrolling participants globally for the ACTION3 study
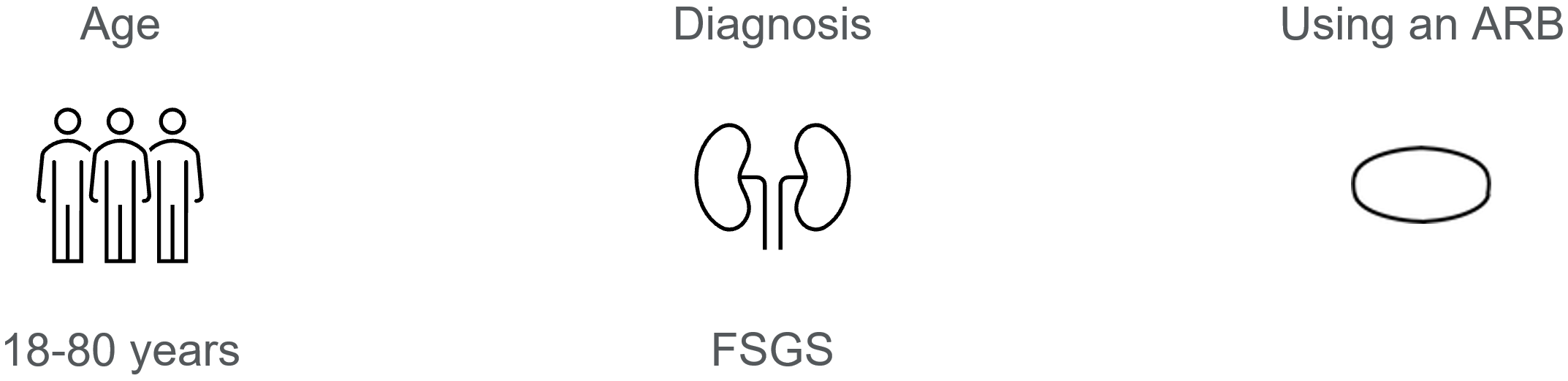
About Focal Segmental Glomerulosclerosis (FSGS)
FSGS is a progressive disease. Progressive means it gets worse over time. When you have FSGS, the filters (glomeruli) of your kidneys become inflamed and are damaged by scarring. This makes the filters “leaky” and allows protein from your blood to collect in your urine (proteinuria).
For patients with FSGS, the kidneys’ ability to purify (clean) the blood is impaired. This can lead to kidney failure that may eventually requires dialysis or a kidney transplant.
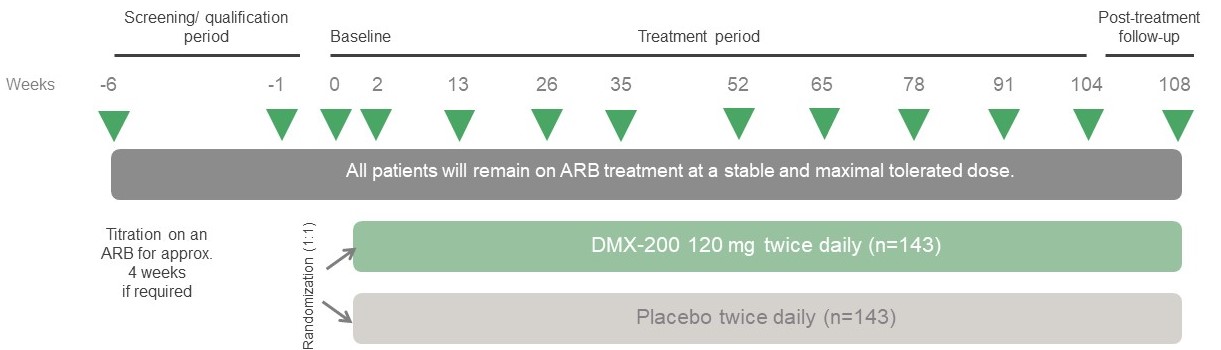
What does the ACTION3 study involve?
The ACTION3 Study will investigate to see if the investigational medicine, DMX-200 (repagermanium), reduces the amount of proteinuria (when protein from your blood collects in your urine) and slows the decline of kidney function, when taken in addition to a medicine called an angiotensin II receptor blocker (ARB). An ARB is a very commonly used medicine in FSGS. It will also investigate what side effects may occur.
The ACTION3 study will compare the investigational medication with a placebo to see if it works better. The placebo looks like the study drug but does not contain DMX-200. Half the study participants will receive the investigational medication and the other half will receive the placebo. Patients who receive DMX-200 or placebo will still receive the best standard of care treatment for FSGS during the study. Both the investigational medication and placebo will be given as capsules that will need to be taken twice daily by mouth. Neither you nor the study team will know if you are receiving the study drug or the placebo.
This is a global study involving approximately 300 patients. The study will last up to 2 years and 3 months and will involve 13 study visits at which your doctor will assess your health and the effectiveness of DMX-200.
If the study is right for you and you choose to participate, all study-related care and study medication will be provided at no cost during your participation.
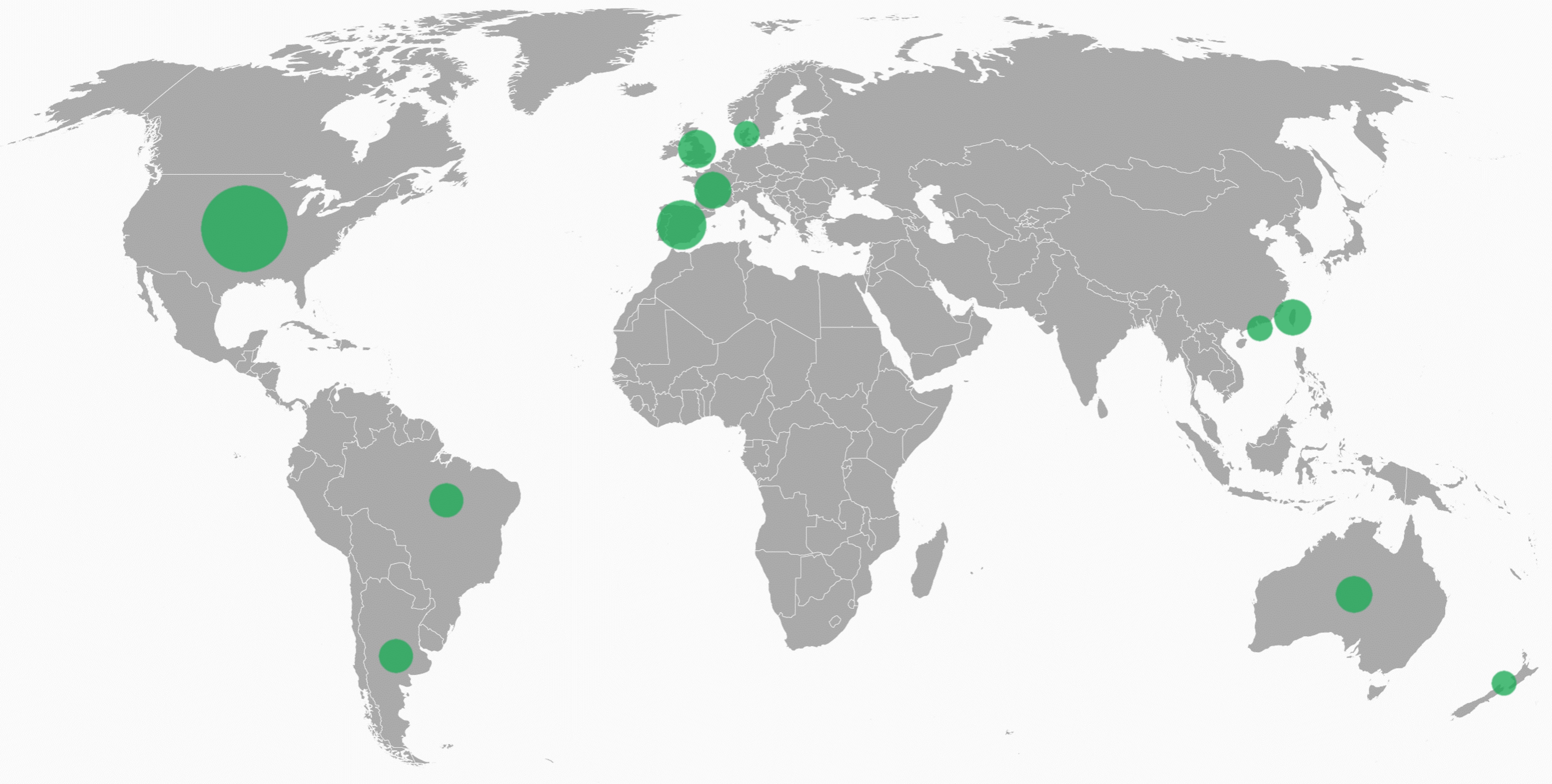
Participating sites/hospitals
The ACTION3 trial is being conducted in over 70+ sites globally.
-
If you are interested in participating, please contact your treating physician and request they contact Dimerix at ACTION3@dimerix.com for more information on your closest participating site.
-
If you are a nephrologist with any potential patients, please contact us at ACTION3@dimerix.com.
The size of the circle indicates the total number of trial sites open in each country.