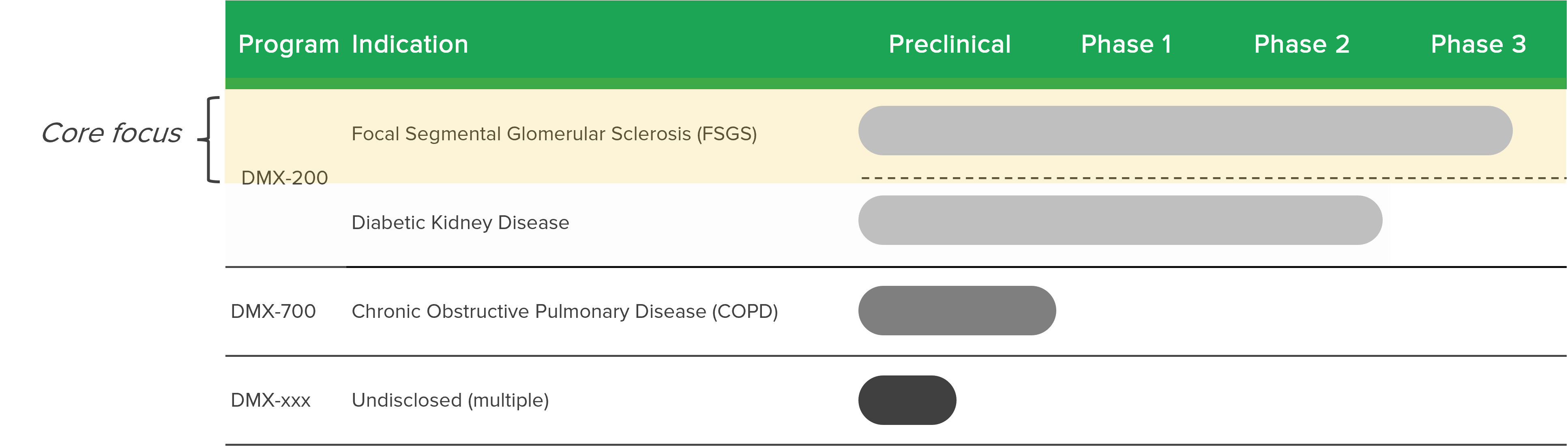
Dimerix pursues new product concepts and applies strong scientific know-how to the discovery of products from early stage development through to commercialisation. Dimerix products will target multiple global territories.
Dimerix strives to develop products to help patients with unmet medical needs and the investment in research and development includes the use of state-of-the-art technology and collaborating effectively with Dimerix partners to help those patients most in need.